DNA phosphorothioate (PT)-modification, with a non-bridging oxygen in the phosphodiester backbone substituted by sulfur, is an epigenetic marker in prokaryotes and is involved in the bacterial defense system, anti-oxidative stress, and gene regulation. PT-modification is specifically recognized by sulfur binding domains (SBDs) of PT-dependent restriction endonucleases, making it a potential tool for enabling biotechnology development. However, the unclear recognition sequence-range of SBDs limits the application.
A study published in the journal Science Bulletin was led by Prof. Lixin Zhang (State Key Laboratory of Bioreactor Engineering, and School of Biotechnology, East China University of Science and Technology) and Dr. Guang Liu (State Key Laboratory of Microbial Metabolism, Joint International Research Laboratory of Metabolic & Developmental Sciences, School of Life Sciences & Biotechnology, Shanghai Jiao Tong University).
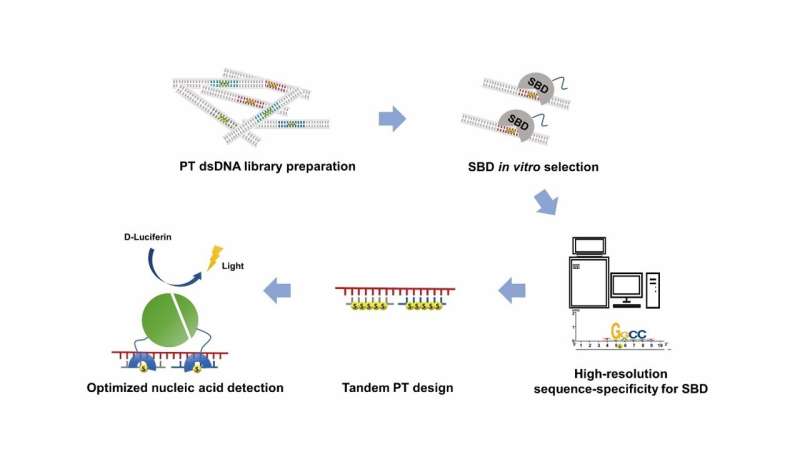
This study reported a technique named sulfur binding specificity-sequencing (SBS-seq) for high-resolution characterization of the complete sequence-range of SBDs, and employed tandem PT-modifications to improve the binding affinity and extend the sequence-range of SBDs. The strong affinity and broad range facilitated a new nucleic acid detection platform based on SBD and PT-DNA.
In order to profiled the high-resolution complete sequence-specificity of SBDs, Yuting Shuai et al. developed the SBS-seq technique, which involves four core steps: (1) the construction of an unbiased random PT-DNA library, with the randomness of the constructed PT-DNA library confirmed by deep-sequencing; (2) in vitro binding selection with SBD protein; (3) verification of the bound substrate by electrophoretic mobility shift assay (EMSA); (4) deep-sequencing and data analysis. SBS-seq results were further validated by fluorescence polarization (FP) assays and EMSA, and the mechanism was explained by molecular dynamics (MD) simulations.
They also found that SBDs have stronger binding efficiency on DNA with tandem PT-modifications, and demonstrated that tandem PT-modification design further expanded the sequence-range and improved the binding affinity of SBDs.
SBS-seq identified the high-resolution complete sequence-specificity, and tandem PT-modification design further expanded the sequence-range and improved the binding affinity of SBDs. Based on these results, they facilitated the SBD based nucleic acid detection system for the detection of ssDNA.
In conclusion, this team developed a high-resolution deep-sequencing-based technique SBS-seq for measuring the complete sequence-specificity of PT-DNA binding SBDs, and extended the sequence-recognition spectrum of SBDs and enhanced the binding affinity by adding tandem PT-modifications in DNA.
Based on these results, they improved the detection sensitivity of SBD and PT-DNA based nucleic acid detection platform. Their work provides insights for research on modification-dependent restriction endonucleases and facilitates biotechnology applications of PT-modification.
More information:
Yuting Shuai et al, Profile and relaxation of sequence-specificity of DNA sulfur binding domains facilitate new nucleic acid detection platform, Science Bulletin (2023). DOI: 10.1016/j.scib.2023.07.012
Citation:
Facilitating a new nucleic acid detection platform (2023, September 29)
retrieved 29 September 2023
from https://phys.org/news/2023-09-nucleic-acid-platform.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
