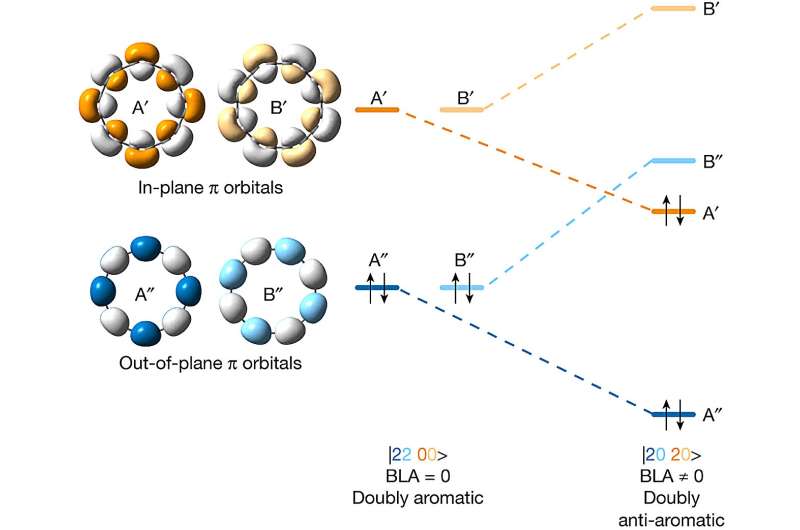
A combined team of chemists from Oxford University, and IBM Research Europe-Zürich has successfully synthesized a doubly anti-aromatic C16 carbon allotrope. In their paper published in the journal Nature, the group describes their synthetic process and its possible use in exploring new experimental theories.
In 2019, a different team of chemists synthesized a new allotrope (molecular form) of carbon, C18—it was made in circular form with the atoms linked by alternating triple and single bonds. The achievement suggested that it might be possible to synthesize a C16 carbon allotrope. Prior efforts had failed due to the inherent instability of the process. In this new effort, the research team has overcome problems faced by others to successfully create a doubly anti-aromatic C16 carbon allotrope.
To achieve their feat, the researchers realized that they would need to overcome the instability of the precursors because they are anti-aromatic due to their higher energies. Their approach involved using several masking agents to improve stability.
The final synthesis process involved using a macrocycle that had two bromine and four carbon monoxide substituents, the results of which were laid on a substrate of sodium chloride. The team then used a tunneling microscope to send picoamperes of charge to specific sites on the molecule. This pushed up the amount of voltage on the bromine to 1.3V and the carbon monoxide to 3V—enough to force bonds to form between the carbon atoms. The result was the formation of a polyyne structured ring held together by alternating triple and single bonds.
The researchers then confirmed that they had created the desired allotrope by focusing on their ring with an atomic force microscope. They also found that they were able to study the distribution of the electrons in the molecular orbitals using a Kelvin probe force microscope and the scanning tunneling microscope. This showed that the molecule was doubly anti-aromatic, as expected.
The work could open the door to new research opportunities involved in the exploration of new chemistry theories and perhaps to the development of new materials made from exotic carbon allotropes.
More information:
Yueze Gao et al, On-surface synthesis of a doubly anti-aromatic carbon allotrope, Nature (2023). DOI: 10.1038/s41586-023-06566-8
© 2023 Science X Network
Citation:
Chemists synthesize doubly anti-aromatic C16 carbon allotrope (2023, October 27)
retrieved 27 October 2023
from https://phys.org/news/2023-10-chemists-doubly-anti-aromatic-c16-carbon.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
